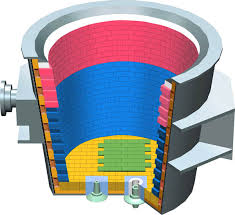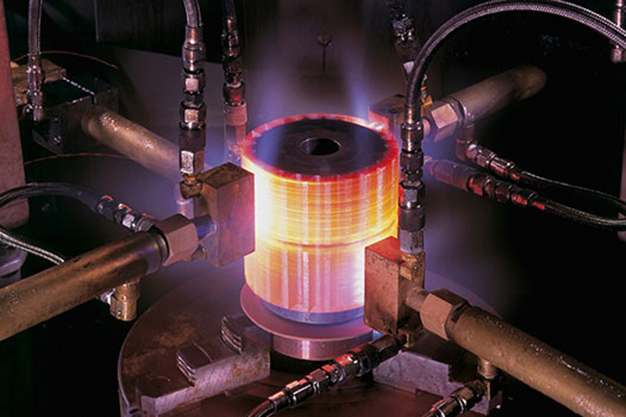
Heat Treatment is the procedure of heating and cooling metals to change their physical and mechanical properties without changing their shape. Basically, it is a mechanism for strengthening materials but could also be used to transform some mechanical properties such as improving formability, machining, etc. This module helps you to understand the different Heat Treatment process that is required to change metals' physical and chemical properties.